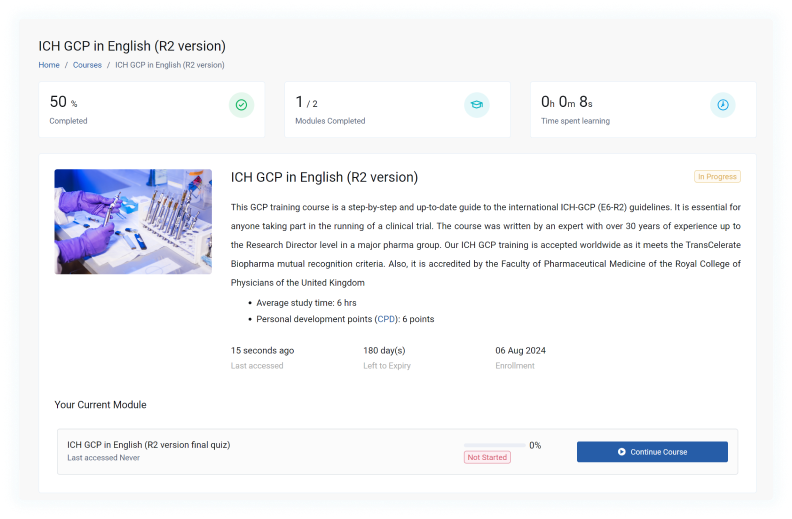
Course Syllabus
- Course Overview
- What are Clinical Trials?
- What is a Placebo Treatment?
- What is ICH GCP?
- What is a CRO?
- What is the Role of the Study Co-ordinator?
- Main Activities
- Ethics Committees
- Ethics Committee Submissions and Approvals
- Institutional Approval
- Competent Authority Approval
- Quality Management
- Investigator / Institution Agreements
- Investigator’s Brochure
- Essential Documents
- Site Training
- Site Initiation Visit
- Patient Recruitment
- Informed Consent
- Randomisation of Patients
- Patient Management & Assessments
- Protocol Deviations
- Investigational Product (Study Drug) Management
- Data Collection
- Electronic Systems
- Adverse and Serious Adverse Events
- Site Monitoring
- Frequency of Monitoring Visits
- Communication with the Sponsor
- Filing and Document Management
- Quality Assurance
- Audits
- The Monitoring Report & Plan
- Research Fraud and Misconduct
- Database Locks
- Study Close Out
- Archiving at the Site
- Glossary & Abbreviations
- Useful Resources
Our GCP certified customers